Document Type : Original Research Paper
Authors
1 Department of Process and Materials Engineering, National Polytechnic School, Oran, Algeria. LTE Research Laboratory, National Polytechnic School of Oran, Algeria
2 Laboratory of Physical Chemistry of Materials, Catalysis, and Environment, U.S.T. Oran, 31000 Algeria
Abstract
The Beni Saf Water Company (BWC) desalination plant in Ain Témouchent (Algeria) uses reverse osmosis technique. This process, which is based on dense membranes operating at high pressure, produces a permeate with characteristics that exceeds the necessary requirements. However, the Reverse Osmosis (RO) technique suffers from the following limitations: high energy consumption, low water recovery and excessive fouling of membranes. We conducted this work to contribute to the study of seawater desalination by introducing another system such as Nanofiltration (NF) to reduce energy consumption and retard the fouling of RO membranes. This research study is focused on the installation of Nanofiltration membranes to desalinate seawater with a salinity of about 35mg/L, in order to protect RO membranes and reduce their fouling. NF was found to be effective for partial and selective desalination of the studied seawater in single or two stages, with lower energy consumption than RO. The ESNA1-LF-LD Nanofiltration membrane is more suitable and competitive compared to SWC4-LD for seawater desalination with a 99% removal rate of multivalent ions. It allows for partial desalination and also allows for the preparation of water for industrial use (cooling....) at twice the pressure and at higher conversion rates (90%). These characteristics provide system designers with new options to reduce the capital cost of the system as well as the operating costs.
Graphical Abstract
Keywords
INTRODUCTION
For a long time, the shortage of fresh water has forced mankind to constantly seek new water treatment methods. Desalination of brackish water and seawater can address the problem of water scarcity that threatens some countries such as Algeria. Currently, the technologies used for seawater desalination are generally based on thermal and membrane processes [1]. Desalination by membrane processes has shown its effectiveness worldwide.
Membrane separation technologies were widely used in the chemical industry, food biochemistry, water treatment, and other fields because of their high rejection rate, environmental protection, and easy operation [2, 3, 4,5,6]. A lot of studies have been carried out at the laboratory scale in the field of desalination of brackish water and industrial effluents [7, 8, 9]. The reverse osmosis process has evolved over the last 40 years with a 44% increase in desalination production capacity worldwide [10]. Another membrane process (Nanofiltration) seems to be increasingly investigated as a replacement for RO in the desalination of certain brackish waters in the years to come. This is mainly due to the nature of its membranes which are more permeable and have lower working pressures than RO ones. Furthermore, for some brackish waters, nanofiltration is a process that allows the production of waters that do not necessarily require re-mineralization or at least require a much lighter post-treatment than in the case of RO [10, 11]. BWC desalination plant in Ain Témouchent (Algeria) uses reverse osmosis technique. The latter is based on dense membranes operating at high pressure to produce a permeate with characteristics that go beyond the requirements [12]. However, the RO technique suffers from the following limitations: high energy consumption, low water recovery, and excessive membrane fouling [13, 14].
The Algerian coastal area has experienced frequent episodes of high rainfall, which causes a high level of suspended solids in the raw water. These suspended solids are mainly of mineral origin and cause problems for the production of drinking water and induce increased operating costs. To this end, in October 2020, the desalination plant replaced the sand of the first filtration step (48 filters) with Filtralite® [15]. The first objective was to reduce rack downtime at high levels of suspended solids and to optimize operating costs by reducing the frequency of backflushing. In addition, this change of medium improves the quality of the filtered water and reduces head losses. Additionally, an improvement in the average quality of filtered water (between 0.3 and 0.5 SDI points) was observed compared to the data for the same period of the previous year. This improvement leads to a reduction in the frequency of cartridge filter replacement (https://www.filtralite.com/en/case_studies/filtraliter-beni-saf-desalination-plant-increasing-production-capacity-and-lowering accessed on September 30, 2022). With this in mind, we have carried out this work to contribute to the study of seawater desalination by introducing another system such as Nanofiltration, which reduces the energy consumed and delays RO membrane fouling.
Materials and Methods
Description of the study area
Beni Saf Water Company BWC seawater desalination plant is located on the Mediterranean on the Algerian coast in the Ain Temouchent region, which has an area of 65,700m2, with a 200,000 m3/d production capacity. BWC has worked since March 2010. The location of the BWC plant is presented in the following map Fig. 1 [16].
Water treatment process at the BWC plant
The water treatment process at the Beni Saf plant consists of a collection system and the pumping of seawater through a single round plug connected to a seawater pump tank by a pipe with a diameter of 2.4 m, submerged at 1,400 m from the coast at a depth of 18 m. The pretreatment of the seawater is performed by sand/anthracite filtration and microfiltration using polypropylene cartridge filters. It is then followed by demineralization using RO. Finally, brine and by-products are removed through an outlet pipe of 1.8 m in diameter at a depth of 8 m below sea level, discharging 2 m above the bottom through a single diffuser (1 m in diameter at an inclination of 45°) Fig. 2 [15]. The total terrestrial and submerged length of the duct is 1,400 m [17].
Physico-chemical characteristics of the raw seawater from the desalination plant of Beni Saf
Table 1 [15] shows the physico-chemical characteristics of the seawater to be treated. This water is characterized by high hardness and conductivity, high sulfate and bicarbonate contents, and is very rich in chloride and sodium ions.
The physicochemical properties of membranes
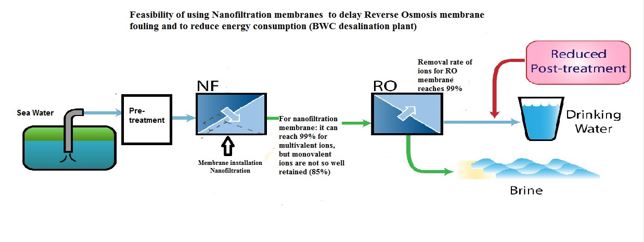
Table 2 shows the characteristics of the membranes used. Fig. 3 shows the simplified process used by the BWC plant to desalinate seawater. The process is divided into three stages: the pre-treatment stage, the reverse osmosis (RO) stage, and the post-treatment stage. Hence, we propose to study the feasibility of installing nanofiltration membranes before the RO membranes to reduce the number of RO modules (replace the 1st pass by nanofiltration modules) to reduce the energy consumed by the RO membrane modules, and thus protect the RO membranes (2nd pass) from solids deposition, which clogs the membranes.
Calculations
Theoretical analysis of the characteristics of the tested membranes was carried out according to the standard membrane transfer methods previously described.
The ion rejection rate is determined by the ratio of the permeate concentration Cp to the initial concentration CO, expressed by the relationship (2.1): [18, 19, 20].
C0: Feed water NaCl concentration (g/l)
Cp: Permeate NaCl concentration (g/l)
The conversion rate (%) is represented by formula (2). It is the quotient of the produced water flow rate by the feed water flow rate.
Permeate flow Jp is determined by relation (3); it is the expression of the permeate flow rate (Qpermeate) about the active membrane surface:
The hydraulic permeability of the membrane Lp: the permeate flow Jv through the membrane is proportional to the transmembrane pressure, as expressed by the formula (4):
Transmembrane pressure (TMP) is expressed by formula (5): it is the driving force that allows transfer across a membrane.
Where:
Pa: Feed pressure (bar)
Pr: Retentate pressure (bar)
Simulation of the Membranes
The IMS Design (Integrated Membrane Solutions design) software provided by “Hydranautics” is the simulation used for dimensioning reverse osmosis and nanofiltration units allowing to achieve the following aims:
- Calculate the number of membranes and modules to be used in the station and choose their type.
- Determining the starting pressure.
- Regulating flow rate and conversion rate.
- Regulating the dose of chemical products.
Simulation of the reverse osmosis membranes of the BWC plant
To simulate RO membranes, the IMS Design software was used following the steps shown in Fig. 4.
simulation of the nanofiltration membranes of the BWC plant
The selected nanofiltration membranes (ESNA1-LF-LD) are designed to provide high rejection of natural organic materials and moderate reduction of total hardness while operating below 100 psi, offering energy and cost savings. Indeed, they offer 50-90% salt rejection with very low operating pressure, increased energy savings, and significantly reduced installation and operating costs. ESNA1-LF-LD are high-performance nanofiltration membranes ideal for water softening, desalination, and removal of pesticides, bacteria, and viruses (https://membranes.com/solutions/products/nf/esna-2/, accessed on September 30, 2022).
The same simulation steps were run using IMS Design software while keeping the same RO process operating parameters, except for the feed pressure, which was varied to 38.6 bar.
Results and discussion
A comparative study was conducted between the reverse osmosis membrane (SWC4-LD) and the nanofiltration membrane (ESNA1-LF-LD).
The characteristics of the nanofiltration membrane given in Table (1) show that the operating pressure of the nanofiltration membrane is half that of the RO membrane. For this purpose, we assessed the salt retention rate by the nanofiltration membrane to study the feasibility of replacing RO membranes with nanofiltration ones to minimize the operating pressure and save energy.
Results of the simulation on RO membranes
Table 4 represents the simulation results of seawater treatment using RO membranes with IMS Design, while Table 5 represents the simulation results of the chemical parameters of the treated water. The simulation results meet the drinking water production standards of the BWC plant.
Simulation results for nanofiltration membranes
First configuration (non-stepped)
To study the nanofiltration membrane, we conducted a simulation using the IMS Design software and kept the same operating parameters as the OI process, except for the input pressure which was set at 38.6 bar. The raw water parameters from the BWC station (Table 1) were entered into the simulator. We also entered the following operating parameters (Table 3): permeate flux, conversion rate, system configuration, number of stages, number of pressure tubes, number of elements/tubes, and membrane selection. Fig. 5 shows the simulation flowsheet of the first proposed configuration. Table 6 represents the simulation results of nanofiltration membranes for the first configuration, concerning monovalent ions, while Table 7 represents the simulation results of nanofiltration membranes for divalent ions. The removal rate of monovalent ions and the removal rate of multivalent ions are represented in Fig. 6 and Fig. 7, respectively.
The term ‘nanofiltration’ signifies that particles of Nanometric dimensions are separated through the NF membranes. NF membranes have low molecular weight cut-offs (200 - 1000 Da) and smaller pore sizes (1 nm). Therefore, the separation of components with these molecular weights from higher molecular weight components can be accomplished [21]. They also have a surface electrostatic charge which gives them great selectivity towards ions or charged molecules. More specifically, the NF membrane can be used to remove small neutral organic molecules while surface electrostatic properties allow monovalent ions to be reasonably well transmitted with multivalent ions mostly retained [22].
Simulation results show that the nanofiltration membrane allows the separation of components in a solution with a size close to the nanometre level. Monovalent ions are not retained by this type of membrane (Fig. 6). On the other hand, multivalent ions (calcium, magnesium, aluminum, sulfates....) are strongly retained (Fig. 7). This type of membrane is very effective in removing small dissolved molecules such as pesticides, humic and organochlorine acids as well as biodegradable dissolved organic carbon (BDOC). The overall passage in salts is 30-60% for monovalent ions and 5-15% for divalent ions.
Comparison of simulation results (IMS Design)
A comparison between nanofiltration and reverse osmosis membranes was conducted. Table 8 represents a comparison of the physical parameters of the simulation results (IMS Design), while Table 9 compiles the comparison of the chemical parameters (IMS Design). For the first configuration, Fig. 8 represents a comparison of the removal rates of monovalent and multivalent ions by reverse osmosis membranes and nanofiltration membranes. From the simulation results, we can see that the removal rate of the nanofiltration membrane varies with the type of ion, while the removal rate of the RO membrane reaches 99% for all ion types.
A nanofiltration (NF) membrane works similarly to reverse osmosis except that with NF, less pressure is needed because of the larger membrane pore size (0.05 μm to 0.005 μm). Nanofiltration can remove some total dissolved solids, but is often used to partially soften water and is successful at removing solids, as well as dissolved organic carbon. For low TDS brackish waters, NF may be used as a standalone treatment for removing salts [23]
Second configuration (stepped)
We entered the following operating parameters (Table 3) into the simulator: permeate flux, conversion rate, system configuration, number of stages, number of pressure tubes, number of elements/tubes, and membrane selection.
A second configuration was proposed to test its effect on the quality of the water produced as shown in Fig. 9. The results of dimensioning for the proposed nanofiltration membrane in the second configuration are presented in Table 10. To compare the first configuration to the second configuration, the removal rates of monovalent and multivalent ions for the two proposed nanofiltration membrane configurations are shown in Fig. 10.
The results of the second configuration (Fig. 10) show an improved ion removal rate, which can reach up to 99% for multivalent ions, and about 85% for monovalent ions.
The removal rate of nanofiltration membrane can vary depending on the performance and initial characteristics of the membrane used, so it depends on the membrane configuration.
The main driving force in a membrane-based seawater desalination process is pressure and desalination cost is then directly proportional to the applied pressure [24]. The quantity of water produced from NF membranes is high but unfortunately, the quality does not meet the standard for either domestic or agricultural purposes [25].
Conclusion
Nanofiltration is a membrane separation technique that lies between reverse osmosis and ultrafiltration. It allows the separation in solution of particles with a size close to the nanometer. The main findings were:
Power consumption for the reverse osmosis technique is intimately linked to raw water salinity: the higher the osmotic pressure to be overcome, the higher the pressure to be applied and the higher the pumping energy. On the other hand, for the nanofiltration membrane, the higher the permeability of the salts, the lower the pressure to be applied, and as a result, the lower the required pumping energy.
results show that the removal rate of ions for the RO membrane reaches 99%.
For nanofiltration membrane: it can reach 99% for multivalent ions, but monovalent ions are not so well retained (85%).
The removal rate of nanofiltration membrane can vary depending on the performance and initial characteristics of the membrane used, so it depends on the membrane configuration.
The nanofiltration separation technique can replace RO membranes in case the water is intended for industrial use such as cooling processes to minimize energy consumption: the nanofiltration membrane consumes half the energy consumed by the RO process. It can be used for partial and selective desalination and wastewater treatment to minimize energy consumption.
The performance of NF processes cannot meet drinking water standards due to their inability to reduce the salinity of seawater to acceptable levels. However, NF can be used before the RO process since divalent ions such as calcium, magnesium, and sulfate ions present in seawater, which constitute a major source of scaling in the RO process, are strongly rejected.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interest.